I have been searching online trying to get a feel for how much salt is in a salt pool. I know that it’s about 3500 parts per million, but what is that really?
So I run across a couple of pool sites that had me laughing (more so than many of the others). There is so much bad and misleading information.
One says: “Chlorine is created naturally by electrolysis”. Look at the insert in the screenshot below.
Naturally by electrolysis??? Only if you’ve just been hit by lightning! And if you have just been hit by lightning I don’t really think you care that your hair is silky soft.
Laughing
And the information found in this screen shot is just wrong.

“hypochlorous acid (chlorine) is converted back to salt”
First of all: hypochlorous acid is not chlorine in either chemistry or name. Secondly: if hypochlorous acid was just converted back to table salt it really wouldn’t poison everything in your pool. Thirdly: chlorine would gas off into the atmosphere.
Going to Wikipedia:Sodium_hypochlorite
NaClO solutions, the following species are thought to be present when the system is in equilibrium.[30] [snip] The free chlorine is thought to be modulated by pH and NaCl as indicated by mercury-chlorine byproducts.
HOCl ↔ H+ + OCl−
HOCl + Cl− + H+ ↔ Cl2 + H2O
The ratio of Cl2 : HOCl : OCl− is pH dependent.[31] The above equations show that the “byproduct” Cl− ions (from the NaCl) play a rarely mentioned role, without them there would be no available chlorine in the solution.
The last sentence is critical.
Cl− ions (from the NaCl) play a rarely mentioned role
The salt in the pool contributes to the generation of chlorine gas from hypochlorite.
Yikes!!!! This would explain the fact that galvanized pool fittings corrode badly in a salt pool. A lot of pool companies websites say that there is not enough salt to affect metal. But turn around and admit that corrosion of metal fittings around the pool is a common problem with a salt pool.
I would bet that it’s not the salt concentration it’s the chlorine gas being generated. Chlorine gas is incredibly corrosive. And if your metal components are corroding, so are you. And what is it doing to the plants surrounding your pool? Or the paint on your house? Or your dog? Cat? Kids?
So back to my original question: how much salt is in a salt pool?
Well it’s about 60 millimolar. And I finally found a graph showing the effect of increasing salt concentration on plants. They did a pretty good job with trying to take into account the chloride and sodium contributions of the various media components so I think this is a pretty good study. 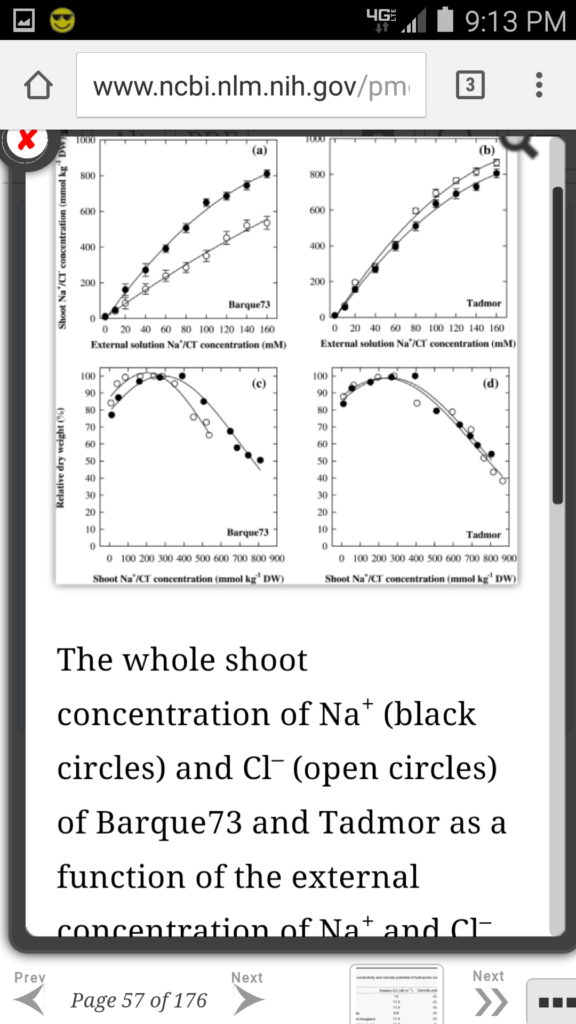
The point is that for the crop plants tested, it is safe to use 60mM salt (your salt pool water) for irrigation (of course only once the chlorine generator and the chlorine gas has been removed :-) ). And, especially if you are irrigating using rainwater in addition to the water from your salt pool. Or, better yet, also using your pool to collect rainwater.
Bob
